INTRODUCTION:
Through successive investigations
over a period of almost a century, on organisms as diverse as plants on one
hand to mammals on the other, it has now emerged as a fact that the extent of
genetic recombination varies from individual to individual in a population and
also during the lifetime of an individual (Bridges 1915, 27). Having conceived this fact, an
obvious question is: What are the mechanisms that govern or affect this
variation?
It was a statistically significant difference in the amount
of crossing-over, in the “black, purple and curved” region
of the second chromosome, between two three-point crosses of the same stock of Drosophila
melanogaster that encouraged Harold Plough (1917) to conduct his classical,
pioneering investigation of the factors affecting the extent of crossing-over.
Plough tentatively attributed that difference in the extent of recombination to
differences in the temperature, humidity or the quality of food that might have
been different in those two cultures of flies. However, when he conducted an
in-depth investigation, he found only the temperature and the age of the female
fly to significantly and consistently affect the extent of recombination in the
offspring of the affected females. Factors such as moisture, starvation,
increased fermentation of food, and increased concentration of iron salts were
found not to cause a significant change in the amount of crossing over.
Plough (1917) found striking increases in the recombination
frequencies in response to temperature changes both above and below the ‘range
of optimal temperatures’ within which the amount of recombination was least
affected. These optimal temperatures ranged from 19ºC to 27ºC. The following
graph taken from Plough’s 1917 paper very clearly depicts changes in the
recombination frequency between the black and purple loci on the
second chromosome, a region spanning approximately 5 map units:
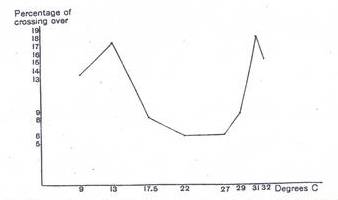
Figure 1: Relation between the amount of crossing over and
temperature for black-purple region on the second chromosome. (Taken from Plough, H.,
1917)
This
U-shaped curve shows that recombination increases with both an increase as well
as a decrease in the optimal temperature range. However, the ‘U’ droops down at
both extremes to form an ‘M’, presumably due to the denaturing effect of
extreme temperature on the enzymatic machinery responsible for crossing over.
In the black and purple region,
recombination frequency increased by as much as 79% for a 9ºC rise in
temperature. Likewise, for a 9ºC fall in temperature, the RF also increased by
73%.
The temperature extremes to which the flies were subjected in this study must have been quite stressful since both the extreme heat and the extreme cold were found to significantly decrease the viability of the next generation. At temperature as low as 5ºC, only a few pupae were formed and no flies ever eclosed, while at a high temperature of 35ºC, although a few females eclosed, but they were all found to be sterile during the first ten days of eclosion.
Plough further discovered that the temperature-induced increase in RF (manifested in the offspring of treated females) was not permanent and faded away with time as the female continued to lay eggs. Furthermore, it was found that the number of days the female was subjected to temperature stress was faithfully reflected in the number of days for which the female laid eggs that yielded a higher proportion of recombinant progeny (that is higher RF). This led Plough to theorize that the developing eggs must be sensitive to temperature changes at a particular stage in their development, and once all the affected eggs have been laid, the later eggs yield the default extent of recombination. This temperature-sensitive-recombination stage can be perceived as the phase in oocyte development during which crossing over actually occurs. While the time it took from the induction of heat stress to the depiction of this stress at full scale in the progeny can be perceived as the period when temperature-sensitive-recombination occurs. Further, studies revealed that the stage that was sensitive to temperature stress is the period after the last oogonial division up to the completion of the prophase of first meiotic division, a period roughly less than two days (Plough 1917). This hypothesis was consistent with the cytological evidence of around 130-140 oocytes that had just passed the last oogonial stage and had begun the prophase of first meiotic division. A female lays the same number of eggs within two days, which is the minimum time it takes for the effect of temperature stress to be expressed at full extent in the progeny (the highest proportion of recombinants for a particular temperature change).
Later, Plough (1921) extended his study to chromosomes 1 and 3. In contrast to the promising results he obtained for chromosome 2, chromosome 1 did not yield any significant changes in recombination due to temperature stress. However chromosome 3 yielded significant results for temperature stress only in a region less than half the size of the entire chromosome. He had already come across some temperature-unresponsive regions in his study on chromosome 2 also, but then he attributed the lack of significant increases in recombination to the greater length of those regions, compared to the regions that were temperature-responsive. He argued in his 1917 publication that the lack of significant increases in recombination due to temperature stress in the non-responsive regions was because of the lack of marker loci in those regions that could register such an increase. However, for chromosomes 1 and 3, the amount of recombination did not increase despite the presence of marker loci in the region. Even more interesting was the finding that almost all the temperature-unresponsive regions were found to have considerably low coefficient of coincidence of double crossovers that is less than 30% or 0.3. It requires further investigation to explain the correlation between low coincidence values and lack of increase in crossing over due to temperature stress.
Throughout the time many different researchers have found an interesting correlation between the age of the mother and the number of recombinants among the offspring in Drosophila (Plough 1921, Bridges 1929, Neel 1941 etc.). The following graph taken from Neel, J. V.’s 1941 article gives an excellent comparative depiction of the effect of the maternal age on the frequency of recombination among the offspring. The graph shows the results for different regions of the third chromosome with different markers used in each case, on the same plot:
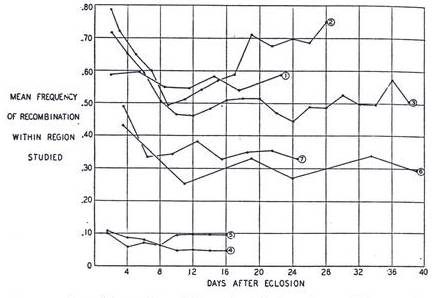
Figure 2: A comparison of the variation in the amount of
crossing over, with the age of the female (mother). Each of these seven curves
represents a different study that covered a different section of the genome and
was conducted by a different investigator in a different year. (Taken from Neel, J. V.,
1941)
It is easy to conclude from the above graph that the extent of recombination is the highest among the offspring from the eggs laid in the earliest days of the life of the mother. However the amount of recombination steeply declines during the first week to a range with very narrow bounds, for the rest of the life.
In an attempt to find a correlation, if any, between larval malnutrition and the amount of recombination in Drosophila, Neel (1941) subjected the larvae to extreme nutritional stress, and contrary to Plough’s (1917) preliminary studies he found a significant increase in the amount of recombination between the h and es loci on the third chromosome. There can be two possible explanations for the failure of Plough’s (1917) experiment to yield a significant change even though Neel’s (1941) experiment did. First, Plough reduced the amount of food in the bottle to only one-tenth; whereas Neel removed the larvae, away from the food, only 70 hours after the eggs were laid. The larvae in Neel’s experiment were then transferred to moistened tissue paper rather than food. Therefore if we subtract the ~24 hours it took them to hatch out of their eggs, they were allowed to feed only for 46 hours. Hence the amount of nutritional stress in Neel’s experiment was much higher than in Plough’s, and the increase in the amount of recombination persisted throughout the 24 day period of the experiment manifesting the effect of stress in all the eggs that the female laid in that period. The average increase in RF over the period following the first week, for the h and es loci, was from 35% to 39%. Although this is not statistically significant, but if all the chi-squares, from all brood counts, over successive periods, are added together, it turns out to be statistically significant. Therefore in Plough’s case, it might also have yielded similar results if the same approach was used. However the difference in the results might also be attributed to the fact that Plough (1917) conducted his study on chromosome 2, while Neel (1941) did his on chromosome 3. The following graph, taken from Neel (1941), illustrates his results over the entire period of the experiment, and it clearly shows the experimental group to have a sustained-high amount of recombination, except for a brief period in the earliest days of life:
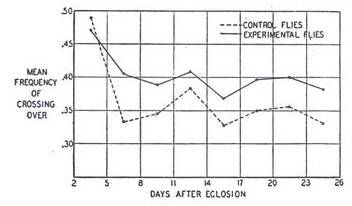
Figure 3: Relation between the age of the female and the variation in the amount of crossing over in the experimental (solid-black curve) and control (dotted curve) group of files, for the h and es region on the third chromosome. (Taken from Neel, J. V., 1941)
Besides the factors (affecting the amount of recombination) discussed thus far, an investigation of the trans-effect of multiply-inverted chromosomes on recombination among marker loci on the ‘other’ chromosomes also yielded an increase in the rate of recombination (Grell 1978). This time however, the effect (trans) of inversions was more pronounced in the regions close to the centromeres, and those close to the ends of the chromosomes. These regions normally have low coefficient of crossing over. The medial regions of the chromosomes (those in between the centromeres and the ends) in contrast were less affected by the presence of serial inversions on a chromosome in the same genome. One possible explanation of this occurrence could be that multiple inversions, that form ‘inversion loops’ during crossing over, might have induced torsional stress in the DNA, which in turn activated cascades of DNA repair machinery, resulting in an overall increase in the activity of DNA repair enzymes that lead to increased recombination. The following graph, taken from Grell’s 1978 article, compares the effect of temperature stress to the interchromosomal effect of the second and third chromosome balancers (chromosomes with multiple inversions) on the amount of crossing over in the X chromosome. Both the balancers were heterozygous, and the amount of crossing over was measured for a region roughly 70 map units. It is interesting to note here that contrary to Plough’s (1921) results for the effect of temperature on the X and the third chromosome, in which he found the X chromosome to be completely unresponsive to temperature stress regarding the amount of recombination, Grell (1978) not only found increased rates of recombination in X chromosome with respect to both temperature stress and balancer chromosomes, but also found the effect to be more pronounced in the regions of lower coincidence values; this again contradicts with Plough’s results of having the higher coincidence regions being more responsive to temperature stress.
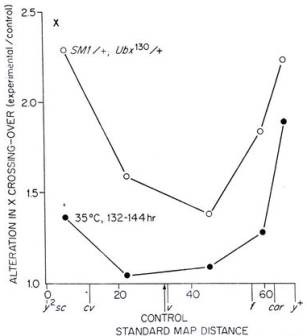
Figure 4: The effect of heat (solid-black dots) and the
presence (trans effect) of balancers (hollow dots) on the amount of crossing
over among five regions on the X chromosome. (Taken from Grell, 1978)
Nonetheless, at least these studies tell us that there is some effect of the presence of heterozygous balancers in the genome, on the amount of crossing over in the other chromosomes, and that the same is true for temperature also.
Despite all the controversies and discrepancies, the key fact to learn from all the studies mentioned thus far is that there exists a considerable range of variation in the extent of crossing over and based on the studies discussed so far, it depends, to a great extent on an individual’s metabolic and physiologic state; any physiologic or metabolic stress can significantly increase the amount of crossing over. Therefore it is not without logic to assume that any genetic deficiencies that generated the same or similar kind of physiologic and/or metabolic stress would also produce similar increases in recombination frequencies as observed for different environmental factors.
However, before we turn to genetic basis of variation in RF, let us turn our attention away from Drosophila to see if there have been any reports of similar changes in RF in other organisms also.
Remarkably, many other organisms have also yielded similar responses to various environmental stresses as observed in Drosophila. To start with a simple eukaryote; certain hotspots for recombination were recognized in the yeast genome through different studies (Abdullah & Borts 2001). Of all the different hotspots, the most studied are those capable of docking a wide-spectrum transcription factor (Gcn4p), regulating the biosynthetic pathways of certain amino acids and purines. It was believed that the transcription factor manifested its effect on recombination through chromatin remodeling, which is part of its job as a transcription factor. Certain transcription factors are believed to be able to recruit chromatin-remodeling enzymes such as histone acetyltransferases. The subsequent exposure of nucleosome-free DNA is believed to allow the recombination machinery to act on it. Moreover, some of these transcription factors might also directly interact, in some way with certain enzymes involved in DNA recombination. The gene (GCN4) for the transcription factor (Gcn4p) itself is regulated by the availability of the end product of the synthetic pathways it runs, that is by the presence of leucine, lysine (amino acids) and adenine (a purine), to name but a few.
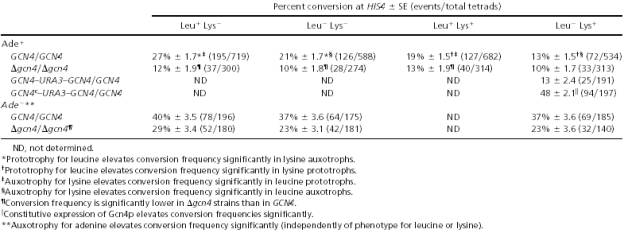
In order to further investigate the effect of the above-mentioned nutrients on the expression of GCN4, and the effect of Gcn4p on the amount of crossing over at the HIS4 locus, Abdullah & Borts (2001) used Ade-, Lys- and Leu- auxotrophs to simulate the lack of adenine, lysine and leucine respectively in yeast diploid cells bearing either the wildtype (GCN4) or the deleted (Δgcn4) version of the transcription factor gene. They found the lack of adenine to significantly increase recombination regardless of the presence of lysine and leucine. The lack of lysine also produced similar, although less intense increase in recombination.
However, the lack of both adenine and lysine failed to significantly increase recombination in individuals with the deleted (Δgcn4) version of the gene. On the contrary, individuals with the synthetic, constitutively active, version of the gene (GCN4c) showed far higher rates of recombination than the wildtypes (see the following table).
Table 1: The effect of the lack of
some key nutrients (adenine, leucine and lysine) on the amount of crossing over
in yeast, at the HIS4 locus. (Taken from Abdullah & Borts 2001)
Among the higher eukaryotes, a study reported stress-induced increase in genetic recombination in mice (Mus musculus) also (Belyaev & Borodin 1982). The authors subjected male mice, heterozygous for Ra and a loci (chromosome 2) to social stress due to overcrowding. The males were then testcrossed with females homozygous wildtype for the Ra locus, and homozygous recessive (+a/+a) for the a locus. The males were subjected to the treatment for eight hours everyday, over a 10-day period and were mated after another 12 days; they were kept in groups of 30, in cages 50 X 30 X 15 cm, which resulted in frequent aggressive collisions among the subjects. The extent of the stress can be perceived from the fact that such same sex congregations are naturally rare among male mice, therefore the treatment must have been quite stressful. This resulted in an approximately 30%, statistically significant increase in the RF between the selected loci.
Another interesting study (Lucht et al. 2002) focused on somatic recombination in plants in response to infection by oomycete fungus. The recombination was assayed using inactive constructs of β-glucuronidase (GUS) reporter gene, interrupted by an insertion flanked by sequences of 566 bp homology (see figure); such that if recombination occurred between the homologous sequences, it transformed the inactive reporter into an active reporter gene that could be assayed using 5-bromo-4-chloro-3-indolyl glucuronide as a substrate; which turned blue due to the action of the enzyme.
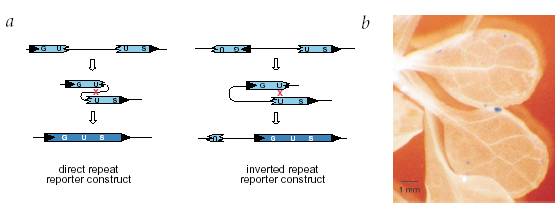
Figure 5: Assaying the somatic recombination events in Arabidopsis
thaliana, in response to pathogen stress using GUS reporter gene. (a) Homologous
recombination between the direct or inverted repeats gives a functionally
active GUS. (b) A GUS positive sector in a leaf stained blue due to the
conversion of a colorless substrate into a blue compound by active GUS. (Taken
from Lucht et al. 2002)
The extent of recombination was determined by counting the number of blue-staining, GUS positive sectors (cells or groups of cells) per plant. Not surprisingly, plants infected with the pathogen showed a statistically significant increase in the number of recombination sectors than the control set (see figure).
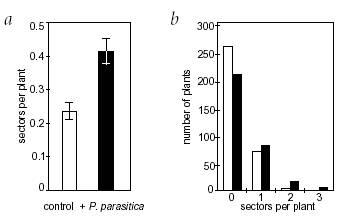
Figure 6: A comparison between the control (uninfected by
pathogen) and experimental (infected with the pathogen) set of plants with
respect to the amount of crossing over. (a) Mean number of recombination sectors per
plant in the experimental & control groups. (b) Number of plants in
each group with a certain number of sectors. (Taken from Lucht et al. 2002).
Since this experiment relied on a transgenic construct for the study of the recombination rate, rather than the plants own genome, it is an excellent demonstration of the correlation between physiologic stress and genetic recombination. It clearly indicates that at least in plants, stressful conditions result in a global upregulation of the enzymes responsible for genetic recombination.
In yet another study, Zhuchenko et al (1986) found a correlation, between the amount of crossing over and changes in temperature, in tomato, exactly the same as that recorded for Drosophila by Plough (1917). This time the curve was V-shaped with minimum crossing over at a temperature of 23˚C, and the two high points at 12˚C and 32˚C respectively. Another interesting finding from the same study was the correlation between the mass of seedlings and the amount of crossing over in their parents. This relation was negative such that the heaviest seedlings came from the parents with the lowest RF, and the lightest came from the parents with the highest RF. This finding establishes a link between the genotype and the amount of recombination, with good genotypes undergoing minimal recombination as compared to the bad ones. However, there can be different strategies to fitness, the seemingly light-seed-producing parents might have produced far more seeds than those that produced heavier seeds. Therefore further investigation is needed to support the above stated conclusion.
Nonetheless, tomato is not the only organism for which a correlation between fitness and recombination has ever been recorded. In a study focusing on the relation between fitness and recombination in Drosophila, Tucic et al (1981) also found a negative correlation between fitness and the amount of genetic recombination. The following graph illustrates the relation between fitness and recombination frequency, using the three criteria of fitness: viability, male fertility and female fecundity. Of these criteria, male fertility and female fecundity relate negatively to the RF; with the results being statistically significant for one out of four regions (of chromosome 2) in case of male fertility, and three out of four regions in case of female fecundity.
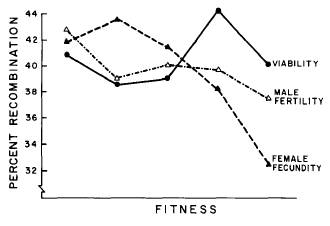
Figure 7: Relation between percent
recombination frequency and the three fitness criteria tested for in the study,
in the 2nd chromosome in Drosophila melanogaster. (Taken from Tucic et al.
1981)
In the light of studies discussed thus far, it is not without logic to draw the conclusion that rate of genetic recombination varies under different physiologic circumstances. Moreover the relation between the physiologic state if an individual and the extent of genetic recombination is negative such that recombination is higher in individuals in poor physiologic state than those in good state. Therefore it should not be difficult to perceive that if certain genetic deficits also give rise to physiologic stress of the same or similar type, RF might also increase as it does in case of environmental stresses.
In our current study, we will attempt to correlate fitness with the extent of recombination in the female parent in Drosophila melanogaster. In contrast to the study on fitness and recombination, just mentioned (Tucic et al. 1981), in which they tested fitness and the extent of recombination for a given 2nd chromosome, in different individuals; we will the same individual for both fitness and recombination. The fitness criterion that we will focus on is the female fecundity. We hope to generate a range of variation in the fitness of wildtype females, by introducing mutagenized balancer chromosomes into their genomes. These mutangenized chromosomes are expected to carry some deleterious mutations, which will decrease the fitness of their carriers. The reason for using mutagenized balancers is that balancers can easily be manipulated, isolated and introduced into other genomes in an intact state, without risking them recombining with other chromosomes in the process. The experimental females carrying a mutagenized 2nd chromosome balancer on wildtype background will be compared, for the extent of recombination in the progeny, to the control females carrying a non-mutagenized 2nd chromosome balancer on wildtype background. This way we expect to negate any differences between the two groups that could arise due to the presence or absence of a balancer in the genome.