|
SLA Batteries |
||||||||
Working with Sealed Lead-Acid Batteries
Introduction
These batteries are known as Sealed Lead-Acid, VRLA (valve regulated lead-acid), or gel-cell. They are similar to car batteries, which are called flooded lead-acid because one can look inside each cell and see the liquid acid solution. VRLA batteries use a gel suspension inside a sealed container (The circular covers over each cell should never be opened). As a consequence, they do not leak, and can be positioned in any orientation. With multiple 2V cells, they are commonly available as 6V or 12V units.
This tutorial deals with how to properly use SLA batteries with only the basic materials: individual batteries, fuses, multimeters, power supplies, and resistors. On the other hand, with expensive charging systems, dealing with these batteries is much more straightforward and requires less knowledge of them.
General Handling and Care
Never allow the terminals to short-circuit, either directly or through a faulty circuit.
- Effects:
- Very high current will flow from the battery, causing sparks, wire meltdown, fire, injury
- Damage to battery and possible explosion
- How to prevent:
- Ensure that all battery terminals are at least 1” apart at all times
- When the battery is in storage, cover the terminals with electrical tape
- Do not store with bits of bare wire or metal nearby
- Never connect any device to the battery without using a fuse. The fuse should be in series with one of the wires to the battery. Preferably, use a covered fuse holder, with a very short wire between the fuse and the battery. Choose a fuse rated at a current which is just a little bit larger than the current you plan to draw from the battery. (Often 1A or 3A for me) If you happen to run out of fuses, please do not cheat by just connecting a wire where the fuse should be.
- Be very protective of the short length of wire between the fuse and the battery. Cover exposed parts of it with electrical tape.
Never give a battery a high impact (eg. By dropping on a hard floor)
- Effects:
- Internal damage (degraded performance)
- Possibly a catastrophic internal short-circuit, leading to fire or explosion
- How to prevent:
- Place batteries in a protective box when transporting
Avoid extreme temperatures.
- Effects:
- Freezing temperatures will cause internal damage, especially if current is flowing in or out of the battery
- High temperatures will slowly discharge the battery and degrade performance.
- How to prevent:
- Do not leave placed on a cold concrete floor
- Keep away from drafty windows in the winter
Lead acid batteries like to be in a high state of charge whenever possible. Never completely discharge. That is, never use a battery until there is no power left.
- Effects:
- Deeply discharging a battery (removing more than 80% of its available power) will cause internal damage – it may never be able to recharge again.
- How to prevent:
- Charge immediately after each use
- Test battery voltage often
- Keep track of how much battery power can be used before a recharge is necessary
- Even if batteries are in storage, they need to be charged back up to 100% every few months
When in use, lead-acid batteries should not be in a gas-tight container.
- Effects:
- When a charge current is filling a battery to near or beyond its capacity, hydrogen gas may be released. This may explode.
- How to prevent:
- When in use, the enclosure should be well-ventilated
- Charge batteries in the open air, if possible
- Keep away from sparks and flames
A sealed lead-acid battery is meant to stay sealed. Do not break it open.
- Effects:
- Hazardous chemicals inside
- The concentrated acid can corrode materials -- even clothing!
- How to treat:
- Flush skin and eyes with water
- Dispose of broken battery, not near flammable materials
Testing
The state of a lead-acid battery can be partly determined by testing its voltage. This test is only accurate if the battery has been disconnected (or switched off) for several hours, preferably 24 or 48. You will need a digital multimeter; an analog one is not precise enough.
|
Voltage of 6V battery |
Voltage of 12V battery |
Percentage of Charge |
Comments |
|
6.37 V |
12.73 V |
100% |
Good |
|
6.31 |
12.62 |
90% |
|
|
6.25 |
12.50 |
80% |
Should be charged soon. |
|
6.19 |
12.37 |
70% |
|
|
6.12 |
12.24 |
60% |
|
|
6.05 |
12.10 |
50% |
|
|
5.98 |
11.96 |
40% |
Must be charged as soon as possible! |
|
5.91 |
11.81 |
30% |
|
|
5.83 |
11.66 |
20% |
|
|
5.75 |
11.51 |
10% |
Battery is damaged or dead (killed) |
|
< 5.75 |
< 11.51 |
< 10% |
The traditional analogy between electricity and water flow can be extended to batteries. We can think of a battery as an aquarium which has the usual width and height, but which is several miles long. The “terminals”, where water is added or drained, are only at one end. The water inside can do work if it is drained out. As a result, the water level will slowly drop, analogous to a falling voltage on the battery. However, the water can’t all be discharged at once, because it takes time for water to flow from the far end to the near end.
If water current is drawn for a while, the water level on the “terminal end” will be at a low level. If we stop drawing current and then test the level again, it will still be low, and we might be led to the conclusion that entire aquarium level is low. But if we wait for a few minutes or hours, water will flow from the far end to the terminal end, and testing the terminal level again will yield the “true” aquarium level.
Conversely, if we “recharge” the aquarium at the terminal end, the level there will be higher there than the rest of the tank, even for a while after charging stops. However, after the water source is disconnected, the level there will slowly decrease until it reaches the level of the rest of the tank.
The moral of the story is: During discharging, battery voltage slowly falls to an artificially low level, and afterwards slowly rises to a stable level. After charging, voltage is artificially high, and slowly falls to stable level. Lead-acid batteries take several hours of being disconnected to reach this stable level.
Discharging
Connecting a battery to a load is termed “discharging”.
The battery’s amp-hour (AH) rating determines the amount of (current * time) the battery can provide. For example, a 10 AH battery can supply 2A for 5 hours or 0.1 A for 100 hours. However, completely discharging a lead-acid battery to 0% capacity will kill it. It is only safe to discharge it to 20%; that is, an 80% discharge. So, for a 10AH battery, it is only safe to actually use 8 AH. Furthermore, it is recommended that these batteries be only 50% discharged, to maintain premium performance.
Prior to use, the battery voltage should be tested to determine the percent charge inside. If a 12V, 10AH battery is measured at 12.37 V, then according to the chart above, it is 70% charged. A 1A discharge current should only be allowed for
(70% - 20%)*(10Ah) / (1A) = 5 hours
Batteries can be connected in series to produce a greater voltage. However, they should have the exact same AH capacity, and should always have the same percent charge. As an example, if two 12V, 2AH batteries were connected in series, we would have a 24V, 2AH "battery".
Batteries can be connected in parallel to produce a greater AH capacity, allowing a larger current and/or a longer time. However, before connecting batteries in parallel, ensure that each is charged to the same voltage level. Each battery should have its own fuse. As an example, if two 12V, 2AH batteries were connected in parallel, we would have a 12V, 4AH "battery".
Charging
Materials:
- DC power supply
- Digital multimeter
- Optionally, a second multimeter (analog or digital)
- Optionally, a high-power resistor (20~100 ohms) to make a pseudo-constant-current source
Cyclic Charge
The battery’s amp-hour (AH) rating determines the amount of (current * time) with which the battery can be charged. Test the battery beforehand to determine the % Charge inside the battery, and thus the additional % Charge it needs, and thus the additional AH’s it needs.
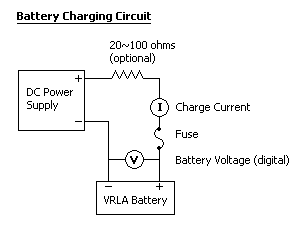
Set the power supply and/or resistor so that a current less than or equal to 1/10 of the battery’s AH rating flows into the battery. Ensure that this current is flowing into the battery, not out. Connect the multimeter(s) so that you can intermittently monitor charge current and battery voltage. The voltmeter’s leads should be connected directly to the battery terminals (or fuse). Voltage may rise above 13V (for a 12V battery) during the charge. Power supply voltage may need to be increased intermittently in order to maintain a desirable charge current.
Disconnect if any of the following conditions arise:
- The required AH’s have passed into the battery
- Battery voltage exceeds 14V (for a 12V battery)
- Charge current falls below ~100mA when voltage is greater than 13.8 V.
- Any unexpected drop or spike in current or voltage
- A cell begins to vent gas.
Periodically, the battery's true state of charge can be determined by leaving it disconnected for 12~36 hours and following the testing procedure already described. By knowing the percentage charge, the estimated time required to charge can be revised.
If, at the beginning of a cyclic charge, the battery voltage quickly rises with a charge current of only 20 mA or less, we say that it is not "accepting a charge current". This is a sign that the battery has been killed earlier by being discharged too deeply.
Batteries can be charged by the same power supply if they are connected in parallel. However, before connecting batteries in parallel, ensure that each is at approximately the same voltage level. Each battery should have its own fuse. As an example, if two 12V, 2AH batteries were connected in parallel, we would be charging a 12V, 4AH "battery".
Trickle Charge/Standby/Float
This method is used if the batteries do not need to be charged quickly, and if you wish to only check their status every day or so. Also, some people leave their batteries on "standby" or "float" after a cyclic charge, to maintain a 100% charge during storage.
Use the same circuit as with a cyclic charge, but with a low-current power supply and/or a current-limiting resistor on the order of 200 ohms. Set the power supply to 12.7 ~ 13.4 V (in the case of a 12V battery). A battery in standby mode must not exceed 13.5 V; this would lead to overcharging.
For more general information on different kinds of lead-acid batteries, see the Trojan documentation:
http://www.taosgreensolar.com/pdf_folder/battery_maint.pdf (PDF format)
http://www.trojan-battery.com/customercare_batterymaint.html (Web format)


