The Haloform
Reaction: Expectations
Read the Experiment.
Complete the Notebook Pre-lab PRIOR to coming to the lab.
(The questions at the end of the procedure are not graded by your TA)
You will not be able to perform the lab without an MSDS section.
BEFORE your lab:
-
Complete the HARARDS AND TOXICITIES and PHYSICAL PROPERTIES
table on the left-side of the notebook.
ONLY include pertinent information ( you do not need to include things
like refractive index, just the information use during the lab)
o Chemicals
that MUST be included are:
§
Benzoic Acid (refer to pg. X)
§ Chloroform
§ Ether
§ NaOCl
§ Sodium Sulfite
§
HCl
§
Acetophenone
- Investigate your compound (benzoic acid) prior to the lab.
- Ask me questions if you get stuck PRIOR to your lab session
- Pre-lab Requirements
o MSDS/Physical
Properties/Outline
o Theory
– Suggested two concepts:
§
Microscale
§
Oxidation
o Purpose
o Reaction with theoretical yield and limiting reagent identified
§
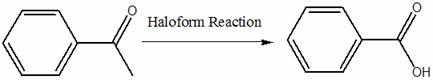
o Reaction
mechanism
NaOCl + NaCl + H2O
→ 2 NaOH + Cl2
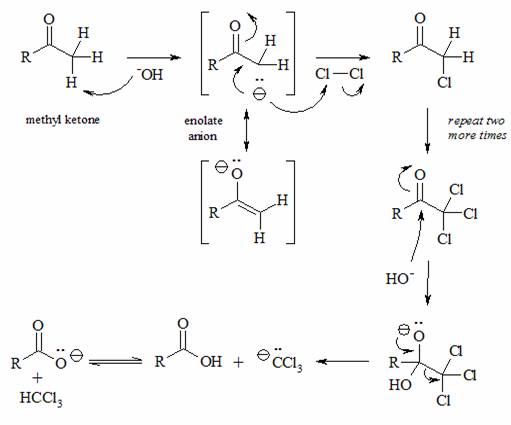
DURING your lab:
-
Reaction 1: Oxidation of Acetophenone
o Using a 5mL conical vial
§ Add 20μL of acetophenone
· Acetophenone has a pungent odour and is a clear yellow -> May cause migraines
· Using 20μL -> 21mg, 0.17mmol of acetophenone
§ Add 700μL of (5%) bleach
§ Clamp vial to a retort stand over a magnetic stirrer
§ Add flea (magnetic stir bar)
§ Attach an air condenser to the vial
· (A reflux setup without water running through the condenser)
§ Turn on stirrer
· This is a hotplate with two knobs, one for stirring and one for heat. Just turn on stirring (slowly increase the RPMs).
§ Mix for 40min at room temperature
· Check occasionally to ensure stir bar is moving
§ Calibrate a 2mL pipette
·
Suck up 2mL of water to calibrate the pipette and mark with a sharpie
or elastic band
§ Remove condenser
§ Add 5mg of sodium sulphite
· This will destroy the unreacted bleach
· After dissolving, add 2mL ether from a calibrated pipette
o Allow layers to separate
§ Upper ether layer is discarded which contains chloroform
§ Bottom aqueous layer
· Contains sodium benzoate
· Use a glass pipette to suck up aqueous layer and transfer to a test tube
o Add 12M HCl (concentrated) to test tube
§ Thick white precipitate forms
§ Congo red turns blue
o Filter
§ Use a Hirsch funnel with tared filter paper
§ Wash with 0.5mL of cold water
·
Benzoic acid is soluble in large volumes of water
o Recrystallize using hot water
o Obtain yield, melting point and IR
-
Hand in your Products in
properly labelled bags (2 bags)
o Bags are present on the TA desk
o Affix a label (also on the TA desk)
§ Fill out all of the fields
·
Name: Yours, including fume hood number
Date: The date you performed the lab
Product: The name of the product
Yield: Theoretical – The
crude mass of your product
Actual The mass after recrystalization % ---> N/A or Theoretical/Actual
*100%
MP: Melting Point that you found. This MUST be a range (XºC -
YºC)
Lit:
The literature melting point of your compound
Colour: Expected -> Look in the CRC or other database for what color your product
SHOULD be
Actual
---> The colour you can see (BTW,
I look and can tell if it isn’t the right colour)
Crystal form: Look it up in the CRC
and write what it should be
Wet/Dry: Write whether your product is wet (a paste) or dry (crystals)
- Remember: Complete a section first and then move onto the next. THINK about the next portion of the experiment prior to starting it.
What are Some Problems I May Run Into and
What Should I Do?
(If suggestions are not EXPLICITY written in the
manual, you MUST ask your TA prior to attempting)
-
My
stir bar is not turning!
o
It
may have gotten stuck
o
Turn
off the stir plate and gradually increase the rotations per minute. If the RPMs is set too high, the stir bar (magnetic flea) can become
stuck.
AFTER your lab:
- Discuss any possible errors which may include:
o Loss of product during recrystallization and state WHY loss would occur.
- Write a final conclusion (~1/2 page) which includes:
o The identity of your compound
o State: (Everything on the product label for both compounds)
§ Mass (in grams), crystal form, name of compound
§ A conclusion of whether your product is pure. i.e. how the above values either enforce or deny your product’s purity.
· It’s OK to say your product was not pure; you will not lose notebook marks for that conclusion. You just need to explain why your product is or is not pure.
§ State whether the compound is pure by commenting on the melting point, crystal form, and colour
o
IR Analysis:
§ Look at acetophenone and benzoic acid IR spectra
§ See what peaks are present for acetophenone and disappear with the emergence of benzoic acid.
§ A clean Benzoic Acid IR will identify that you do indeed have benzoic acid as a product, but does not necessarily mean that it is 100% pure.
Disclaimer: This
document in no way represents the
The author is not responsible for any injury incurred by the webpage or site in general.