Reduction of Aceetophenone using
Sodium Borohydride
Read the Experiment.
This one is a bit more complicated and there are many new
techniques.
Complete the Notebook Pre-lab PRIOR to coming to the lab.
(The questions at the end of the procedure are not graded by your TA)
You will not be able to perform the lab without an MSDS section.
>>>> SAFETY NOTE<<<<
-
Sodium borohydride is caustic. Do not let it come
into contact with your skin. If accidental contact should occur, wash
immediately and thoroughly.
-
Acetophenone – has a very potent smell. Can cause migraines (in most people) and
allergic reaction. ALWAYS cap and use
within the FUMEHOOD.
-
2,4-dinitrophenyl hydrazine – HIGHLY explosive when
dry. Harmful with contact with
skin. DO NOT Touch!
- Make sure YOU and YOUR partner are finished with the heating section (use of hotplates) prior to starting the ether section. Ether is extremely flammable and should not be used with heat sources near by. Take the hotplate out of the fumehood before starting work with ether.
BEFORE your lab:
-
Complete the HARARDS AND TOXICITIES and PHYSICAL
PROPERTIES table on the left-side of the notebook. ONLY include pertinent information
– NB: This lab WILL need
the Refractive Index
o Chemicals that MUST be included are:
§
Sodium
Borohydride
§
Acetophenone
-> DOES NOT GO DOWN THE DRAIN ->SMELLS!!!
§
Ether
§
2,4-dinitrophenyl
hydrazine
§ 1-phenylethanol
§ Magnesium Sulfate
§ Ethanol
- Investigate your compound prior to the lab.
o What are the physical properties of acetophenone? 1-phenylethanol?
§
![]()
![]()
![]()
· CRC 89th Edition
- Ask me questions if you get stuck PRIOR to your lab session
- Pre-lab Requirements
o MSDS/Physical Properties/Outline
o Theory – Suggested two concepts (define and how it relates to this lab exercise):
§ Reduction
§ DNPH Test
o Purpose of this exercise
o Balanced Reaction with theoretical yield and limiting reagent identified (*4 acetophenone/1-phenylethanol)
§
Reduction of Acetophenone
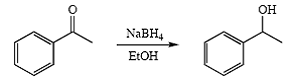
Acetophenone 1-phenylethanol
§
DNPH Test
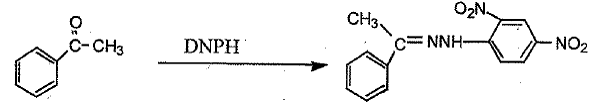
Acetophenone 2,4-dinitrophenylhydrazine 1-(2,4-dinitrophenyl)-2-
(1-phenylethylidene)hydrazine
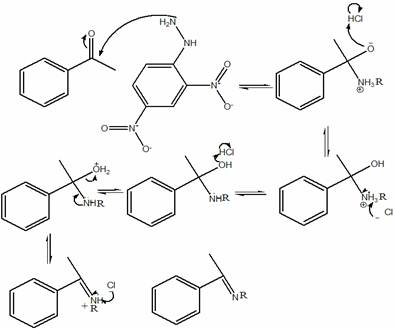
o Reaction mechanism
§ The one state in the lab manual
·
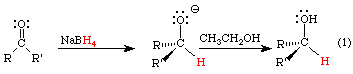
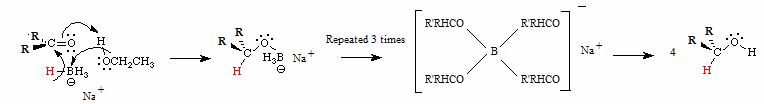
**YOU MUST KNOW THE STEREOCHEMISTRY
§ DNPH Test
·
Acetophenone with DNPH
DURING your lab:
- Reduction of Acetophenone
o Obtain a microscale kit
§ 5mL flask (use which ever one is in your kit)
· clamp to a retort stand
·
Add 0.1g of sodium borohydride
*Do NOT leave out, exposed to air, for a prolonged time
· 1.5mL of 95% ethanol
· Stir until dissolved (uniform, clear [see through])
§ Attach a condenser (air cooled = reflux setup without water)
· *Make sure the condenser is vertical otherwise the dropwise addition will not work and your acetophenone will just coat the condenser tube.
§ Place an ice bath underneath the flask
· Ice bath = ice and water slurry
1. Just ice will insulate the flask due to the air pockets that form from packed ice
2. Water will get into the air pockets and provide a uniform cold temperature
§ Add the 0.6mL drop-wise through a 10-20 minute addition
· *TAs will dispense this amount
· Temperature should be kept between 30-50°C
1. It should feel mildly cool or warm, not ice cold or very hot
1. HOW? Your body temperature is roughly 40°C so keep the temperature around your own.
·
Stir for an additional 10 minutes
*Do the DNPH Test while waiting
§ Add 0.5mL of 3M HCl
§ Heat the mixture using a hotplate
· Approximately 20 minutes
· You should see two layers
§ Cool the mixture using an ice bath
· Make sure your mixture is cold prior to starting the extraction.
·
Ether will boil off at 35°C, so it must be below this temperature
(colder the better)
· Ether is highly flammable, keep away from heat sources
-
Extraction
(keep everything dissolved for proper extraction -> add ether or water as
needed)
o Add 1mL of ether to the vial
§ Mix well
· If you don’t mix the solution well, extraction will not work and very little product will result.
§ Suck up the contents of the vial in a Pasteur pipette
· Wait for the layers to separate
· Squeeze back the bottom layer (should be inorganic/aqueous) into the original vial for another extraction
· The remainder (the organic/ether layer) should be placed in another small, 5mL vial
o Repeat
the above step to perform another extraction
-
o Add 1mL of water to the ether vial
§ Mix well
o Suck up the contents of the vial in a Pasteur pipette
§ Wait for the layers to separate
§ Squeeze back the bottom layer (should be inorganic/aqueous) into the original vial for another extraction
§ The remainder (the organic/ether layer) should be placed in another small, 5mL vial
o Repeat
the above step to perform another wash
- Drying
o Add a few grains of sodium (most likely magnesium) sulphate
§ This will remove any suspended water trapped within the ether
§ Do not add too much as you can loose your product
o Use
a dry pipette to remove your layer and not the magnesium sulphate
- Evaporate your mixture by letting it sit out
o The
product is a liquid which is identified through the use of the DNPH test and by
looking at the refractive index and seeing if it matches the literature value
- DNPH Test
o Test both Acetophenone AND 1-phenylethanol
§ Do this while you are waiting for the reaction to be completed
§ Take a small vessel (test tube) and place 3 drops of acetophenone/1-phenylethanol
§ Add 20 drops of DNPH (2,4-dinitrophenyl hydrazine test)
· Mix well
§ Add 2 drops of concentrated HCl
§ Wait 5 minutes
§ Place in ice
§ Wait 10 min (max)
§ Filter product (if any)
§
§ Do a melting point on the solid
o Since this procedure is very toxic, I usually let one person per row do the experiment and fill out the data sheet and share it with the rest of the group
§
*Do not hand in the product! !!Toxic!!
- Refractive Index *Make sure you record the temperature
o Find out what TEMPERATURE and Refractive Index value of the compounds you are test PRIOR to the lab
o If you do not have a copy of the Appendix, it can be found here
o The refractive is contingent on temperature, so the value at 20°C may not be applicable
§
*refraction for water is much less dependent on
temperature than most organic liquids, decreasing by about 0.0001 for every 1
°C increase in temperature.
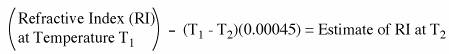
·
T20C = RIobtained – (T –
20)(0.00045)
Estimate of RI you obtained at 20°C = RI obtained – (temperature during RI
acquisition – 20)*(0.00045)
· If you determined the index of refraction of an organic liquid at 24°C, and want to compare it to a literature value determined at 20 °C, you should subtract 4(0.00045) = 0.0018 from the index of refraction you obtained.
- Timeline
o 10min: Quiz
o 25min: Lab Talk
o 20min: Retrieve and setup materials
o 20min: Addition of acetophenone
o 10min: Stir to complete reaction/DNPH test with acetophenone
o 20min: Heat to form two layers
o 10min: Cool mixture
o 10min: Extraction
o 5min: Drying
o 5min: Evaporation
o 20min: DNPH Test with product
o 10min: Refractive Index (the test is short but there may be a line up)
-
Hand in your Data Sheet
o
Either rip out the sheet from your manual or photocopy it
- Remember: Complete a section first and then move onto the next. THINK about the next portion of the experiment prior to starting it.
AFTER your lab:
- Discuss any possible errors which may include:
o Loss of product during recrystallization and state WHY loss would occur.
§ State yield (grams)
§ Compare yield v. theoretical yield
§ You need to state errors if you have less than 80% yield
- Write a final conclusion (~1/2 page) which includes:
o State your objective: Is my compound (1-phenylethanol) pure?
§ Provide proof:
· Colour v. literature
· Refractive Index v. literature
1. Compare refractive indices of starting reagent and product
· Results of your DNPH Test
o
How do you know that you do NOT have your starting material
(acetophenone)?
What are Some Problems I May Run Into and What Should I Do?
(If suggestions are not EXPLICITY
written in the manual, you MUST ask your TA prior to attempting)
- Not all of my sodium borohydride dissolved!
o
That’s okay since an excess of sodium
borohydride is used. Fresh sodium
borohydride dissolves in 95% ethanol, but partially hydrolyzed sodium
borohydride will not all dissolve.
-
How do I take the mass of the product?
o
Since the product is not solid, you will not use
a plastic bag. Just use a glass vessel
to weigh by difference to find out the mass of your product. Vessels you might use are a
watch glass or a test tube included in your microscale kit.
-
How
do I take the refractive index?
o
This
is usually done by your TA because of time restraints
o
If
you were to take one yourself, you would…
§
Open
the prism area
§
Apply
the sample on the black portion (trying to not go too close to the edge
otherwise the compound would overflow)
§
Adjust
the light so the upper field is brightly colour and the lower half is dark
(there might be a red band on the bottom and a blue band on top)
§
Press
the read button and record ALL of the numbers you obtain for your refractive
index value
§
Press
TEMP to find out what temperature it is so you can make the appropriate
corrections
-
What
do I do with my product after the lab?
o
I
can not mark a liquid compound, so it is disposed in the liquid waste in your
fumehood.
-
Why
did I add dilute HCl?
o
It
breaks up the H-B bond
o
Adds
the H to the product
o
Releases
the product
-
My
mixture is not separating during boiling!
o
This
is because the action of boiling is tossing the two layers together. Stop boiling the mixture and see if they
separate. Usually no more than 5 minutes
of boiling is required.
-
The
mixture is still not separating!
o
Try
and add some additional water/ether to your mixture so two layers form (Do not
add more than 2mL of each)
-
Make
sure you do not try to do the pipette separation when solids are present
otherwise the pipette will clog, therefore make sure everything is dissolved
first
Quiz Questions (not necessarily on the quiz)
-
How
do you know that your product has formed if the melting points and boiling
points are so similar?
Support your answer using a DNPH test and Spectroscopic means.
-
Write
the balanced equation for today’s reactions.
-
What
is the mechanism of today’s reaction?
-
What
is the difference between oxidation and reduction?
Disclaimer: This
document in no way represents the University of Toronto at Scarborough. All
opinions and errors are my responsibility as the creator and maintainer of this
page
The author is not responsible for any injury incurred by the webpage or site in general.