Mapping it Out (MRI & PET)
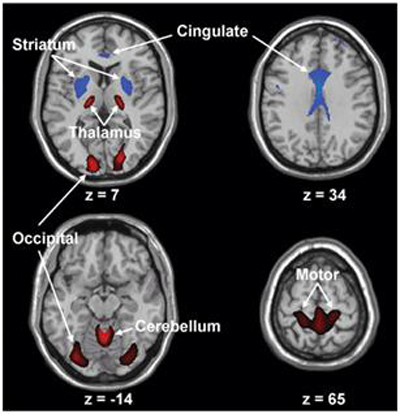 Figure 1. Huntington’s disease is characterized by decreased metabolic activity in the straitum and cingulated cortex (blue), also by increased activity of ventral thalamus, cerebellum, motor cortex and occipital lobe (red).
Figure 1. Huntington’s disease is characterized by decreased metabolic activity in the straitum and cingulated cortex (blue), also by increased activity of ventral thalamus, cerebellum, motor cortex and occipital lobe (red).
Magnetic Resonance Imaging
Both MRI and fMRI are utilized as a method of studying brain atrophies, especially in the region of the striatum, in order to track the progression of the disease in HD patients. It is obvious that there is a higher rate of loss of brain volume over time in the basal ganglia as well as other areas throughout the brain in HD patients as compared to normal individuals(8). Spatial covariance analysis revealed lowered activity of caudate, putamen, and mesial temporal cortex alongside increases in metabolic activity of the occipital cortex (9). At the premanifest stage of phase of HD, T2-w MRI analysis shows an increase in iron accumulation and a decrease in axonal density in the basal ganglia in diseased subjects compared to controls (10). Iron accumulation can be explained by few different hypotheses: 1) iron is known to be transported across the blood brain barrier to diffuse out its high concentration on particular areas of the brain. Axonal degeneration characteristic of HD does not permit the transportation of iron out of the basal ganglia and iron cannot be distributed out; 2) oligodendrocytes release mass amounts of iron, which is later transported to the ganglia, in attempt to repair/remyelinate damaged neurons in HD tissue (10). MRI can be used as a diagnostic tool at preclinical stages and also can assist researchers in discovering different mechanisms underlying the causes of HD.
Position Emission Tomography
Alongside MRI, PET can also be used to determine the onset of symptoms by examining dopaminergic receptor sensitivity. Specialized PET scans have shown decreased binding effects of dopamine on D2-receptors in the striatum in brains of HD patients, which occurs not only at the onset of symptoms but also arises before the initial stages of HD and during the progressive states after the onset (11). Studies have discovered that D2-receptor binding has reduced linearly as the stages of the disease elevated and this was directly related to the decline in cognitive and motor function (12). Scans were performed regularly throughout the stages of the disease to keep track of its development. The scans show further loss of striatal and cortical dopamine receptors roughly four months following the initial onset (13).
