| |
 |
 |
|
 |
How
Solar Cells Work
Converting Photons to Electrons
Photovoltaics (PV) comes from the roots “photo” (meaning “light”)
and “voltaic” (electricity) and its main function
is to convert sunlight into electricity that can power almost
everything from calculators to homes in remote areas to even
satellites.
Current models of PV cells are made from semiconductors like
silicon. When light in the form of photons comes into contact
with the cell, the cell absorbs some of that energy and this
sets the electrons in motion i.e. creating an electrical
current. These electrons can also be restricted into flowing
in a certain direction depending on whether electric fields
are present or not. The addition of metal contacts on the
top and bottom of a PV cell makes the electrical energy collected
from light available in the form of a current.
Silicon in Solar Cells
Silicon is a semiconductor, meaning that it possesses both
metal and non-metal properties. A silicon atom has 14 electrons
so its valence shell contains four electrons that are free
to form bonds with other silicon atoms. Pure silicon forms
a solid, crystalline structure, but the material used in
PV cells contains impurities such as one phosphorus atom
(with five valence electrons) and one boron atom (three valence
electrons) for every million silicon atoms. These impurities
provide free carriers (extra electrons) that can carry electrical
current, and the result is an electric field that only permits
electrons to move in one direction.
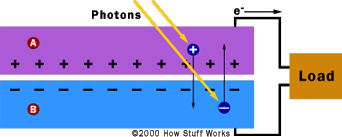 Photons striking the semiconductor material will provide
enough energy to release electrons and start an electrical
current.
Photons striking the semiconductor material will provide
enough energy to release electrons and start an electrical
current.
An antireflective coating is added to the top of PV cells
to fight against the reflection of photons since silicon
is a very shiny material, and this reduces reflection losses
to less than five percent. A glass cover plate also serves
as protection for the PV cell from the elements.
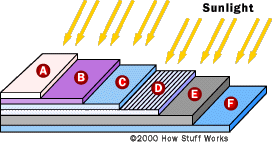
 Basic
structure of a generic silicon PV cell
Basic
structure of a generic silicon PV cell |
Other materials besides single crystal silicon that can be used to manufacture
photovoltaic cells include polycrystalline silicon, amorphous silicon, gallium
arsenide, copper indium diselenide, and cadmium telluride. Sometimes these materials
are used in combination with other semiconductor materials to produce an even
more efficient PV cell.
A typical photovoltaic system also uses deep-cycle batteries (batteries that
can discharge a fair amount of stored energy while maintaining long life) to
store excess electricity that is produced. This is another way to make the cells
more efficient especially on cloudy days when there is less exposure to direct
sunlight.
Credits:
1. Aldous, Scott. How
Solar Cells Work. How Stuff Works, Inc. <http://science.howstuffworks.com/solar-cell.htm/printable>
|
|
 |
|
|
