A Novel Feature and Potential Treatment Area
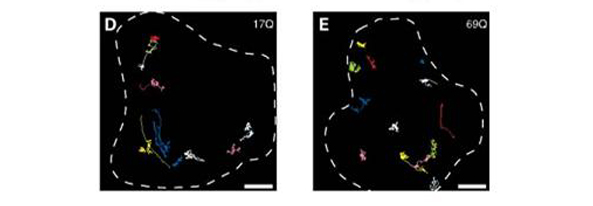 Comparison of the vesicle trajectory between control and cells expressing disease state exon1 of Huntingtin protein in pictures D and E respectively. Increased randomness of trajectories are observed in E shown by the staining of the tubular network that vesicles use to travel. Taken from source 7.
Comparison of the vesicle trajectory between control and cells expressing disease state exon1 of Huntingtin protein in pictures D and E respectively. Increased randomness of trajectories are observed in E shown by the staining of the tubular network that vesicles use to travel. Taken from source 7.
Fluorescence comparison of Cis-Golgi movement.
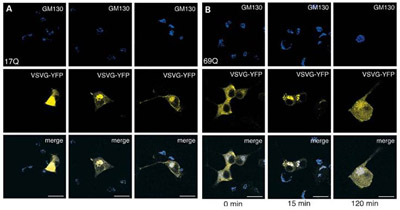 Figure 5. Staining comparison of the vesicle movement from Cis-Golgi to cell membrane. GM130 is a Cis-Golgi marker stained in blue, vesicles travelling through golgi stained with yellow fluorescent protein. Panels in A and B show control and cells expressing exon1 with 69 Glutamine repeats. Taken from source 7.
Figure 5. Staining comparison of the vesicle movement from Cis-Golgi to cell membrane. GM130 is a Cis-Golgi marker stained in blue, vesicles travelling through golgi stained with yellow fluorescent protein. Panels in A and B show control and cells expressing exon1 with 69 Glutamine repeats. Taken from source 7.
Mice cells transfected to express expanded exon1 with 69 Glutamine repeats of Huntingtin gene increased the random orientation of vesicular movement compared to mice cells expressing the same exon with glutamine repeats within normal ranges (i.e. <35 Glutamine repeats) as shown in figure 4 (7). Mutant Huntingtin also seems to interact with β-tubulin through kinesin motors and interfering with intracellular trafficking dependant on microtubules (7). Speed at which vesicles are transported from Golgi to cell membrane is reduced in cells expressing 69 Glutamine repeats of exon1 compared to normal range version shown through figure 5. Comparison of the amount of vesicles leaving the Cis-Golgi inferred from the fluorescence of wild-type (17 Glutamine) threshold and disease state (69 Glutamine) from figure 5 shows that disease state cells have slowed transportation of vesicles from Golgi to membrane shown at 15 and 120 minute mark. Vesicle levels were the same in both control and mutant so the reduced signal in figure 5 was inferred as a reduction in the speed of transport and not lowered levels of vesicles in cell lines expressing 69 Glutamine repeated exon 1 (7).
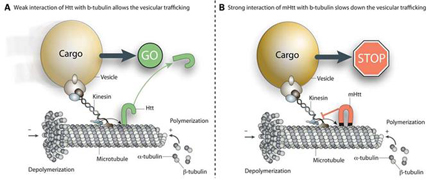
Proposed mechanism of how mutant form of the Huntingtin protein can disrupt vesicular transport on microtubules. Taken from source 7.
Mutant Huntingtin protein has been observed to co-localize with β-tubulin and potentially serve as a physical blockage in axonal transport, figure 6 (7). As mentioned previously, the disease state exon1 alone is sufficient to disturb vesicular trajectory, mutant Huntingtin protein in its entirety may in fact destroy directionality of travel for vesicles depending on microtubules. Neurons depend on axonal transport to deliver vesicles contained with various molecules for communication with other neurons. Axonal transport is dependent in part on the structural integrity of microtubules which is made up of α and β-tubulin. The case for Huntingtin protein and its diseased state version in interacting with intracellular trafficking is becoming clearer, drugs that can prevent mutant Huntingtin from binding to microtubules may reduce Huntingtin’s effect on intracellular trafficking via microtubules.
