

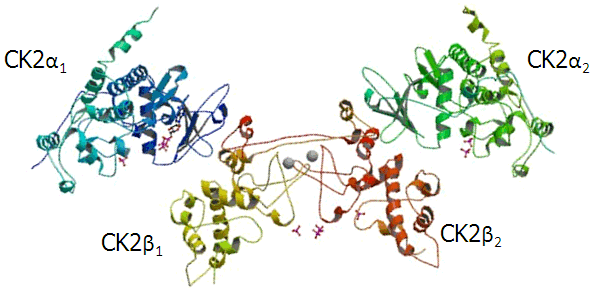
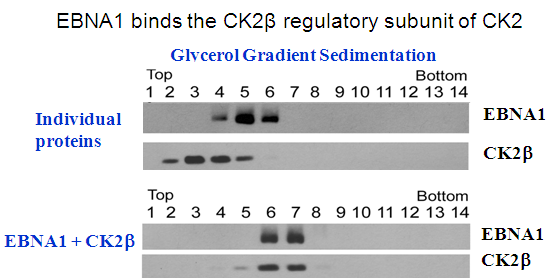

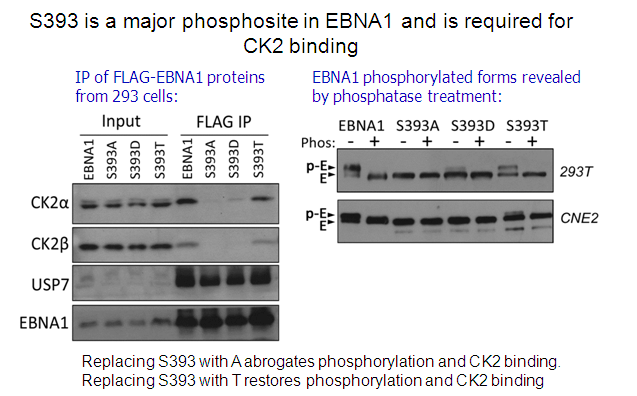
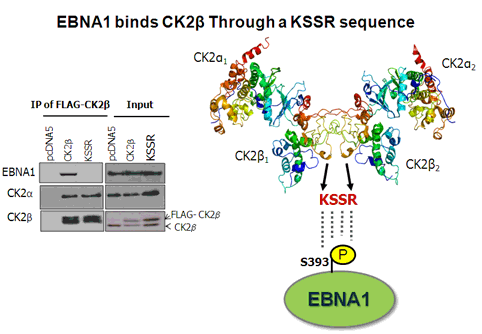
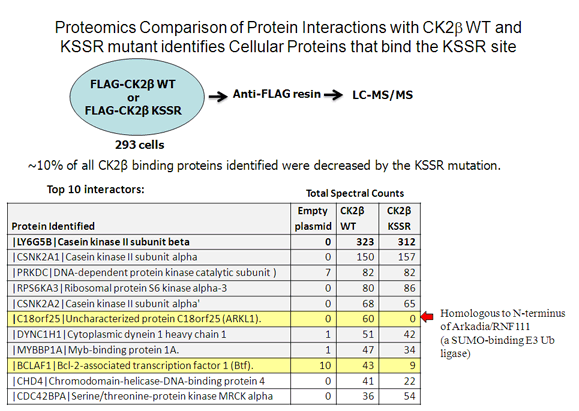
Cao, JY, Shire, K, Landry,C, Gish, G, Pawson, T and Frappier, L. 2014 Identification of a Novel Protein Interaction Motif in the Regulatory Subunit of Protein Kinase CK2 used by Viral and Cellular Proteins. Mol. Cell. Biol., 34, 246-258.
Malik-Soni, N and Frappier, L 2012 Proteomic Profiling of EBNA1-Host Interaction in Latent and Lytic Epstein-Barr Virus Infections. J. Virol. 86, 6999-7002. PMID: 22496234
Sivachandran, N., Cao, J.Y. and Frappier, L. 2010 Epstein-Barr Nuclear Antigen 1 Hijacks the Host Kinase CK2 to Disrupt PML Nuclear Bodies. J. Virol. 84(21) 11113-11123. PMID: 20719947
Holowaty, M.N., Zeghouf, M., Wu, H., Tellam, J., Athanasopoulos, V., Greenblatt, J. and Frappier, L. 2003. Protein profiling with Epstein-Barr nuclear antigen 1 reveals an interaction with the herpesvirus associated ubiquitin specific protease, HAUSP/USP7. J.Biol. Chem. 278, 29987-29994. PMID: 12783858