

Georges, A., Coyaud, E., Marcon, E. Greenblatt, J., Raught, B. and Frappier, L. 2019 USP7 Regulates Cytokinesis through FBXO38 and KIF20B Scientific Reports. 9(1):2724.
Georges, A., Marcon, E. Greenblatt, J. and Frappier, L. 2018 Identification and Characterization of USP7 Targets in Cancer Cells. Scientific Reports. 8(1):15833.
Pfoh, R., Lacdao, I.,Georges, A., Capar, A., Zheng, H., Frappier, L., and Saridakis, V. 2015 Crystal structure of the USP7-ICP0 complex reveals a novel mechanism used by viral and cellular proteins to target USP7. PLoS Pathogens. 11(6): e1004950.
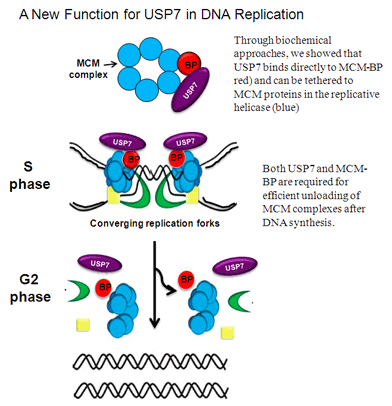
Jagannathan, M., Nguyen, T., Gallo, D., Luthra, N., Brown, G. and Frappier, L. 2014 A Role for USP7 in DNA Replication. Mol. Cell. Biol. 34, 132-145.
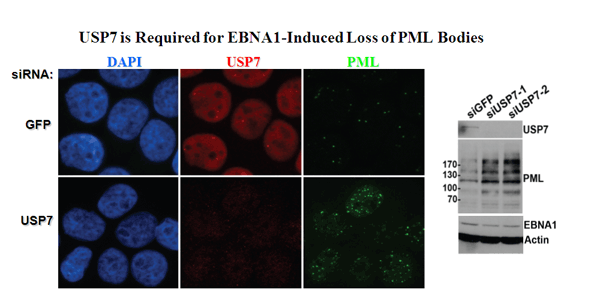
Sarkari, F, Wang, X., Nguyen, T and Frappier, L. 2011 The Herpesvirus Associated Ubiquitin Specific Protease, USP7, as a Negative Regulator of the PML Proteins and PML Nuclear Bodies. PLoS One, 6(1): e16598. doi:10.1371/journal.pone.0016598. PMID: 21305000
Sarkari, F., , Sheng, Y. and Frappier, L. 2010 USP7/HAUSP Promotes the Sequence-Specific DNA Binding Activity of p53. PLoS One. 5(9): e13040. doi:10.1371/journal.pone.0013040. PMID: 20885946
Sarkari, F., La Delfa, A., Arrowsmith, C.H., Frappier, L., Sheng, Y. and Saradakis, V. 2010 Further Insight into Substrate Recognition by USP7/HAUSP: Crystal Structures and Biochemical Analysis of the HdmX and Hdm 2 Interaction with USP7/HAUSP. J. Mol. Biol. 402, 825-837. PMID: 20713061
Sivachandran, N., Cao, J.Y. and Frappier, L. 2010 Epstein-Barr Nuclear Antigen 1 Hijacks the Host Kinase CK2 to Disrupt PML Nuclear Bodies. J. Virol. 84(21) 11113-11123. PMID: 20719947
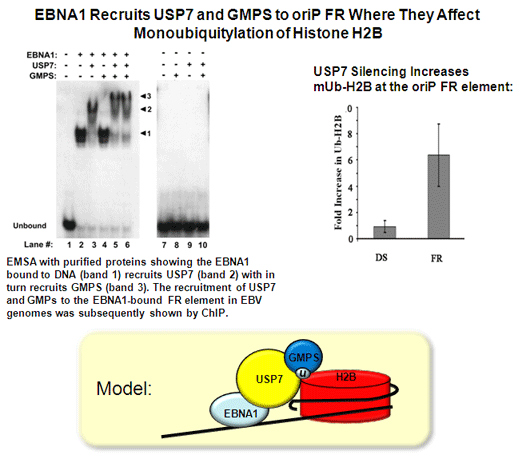
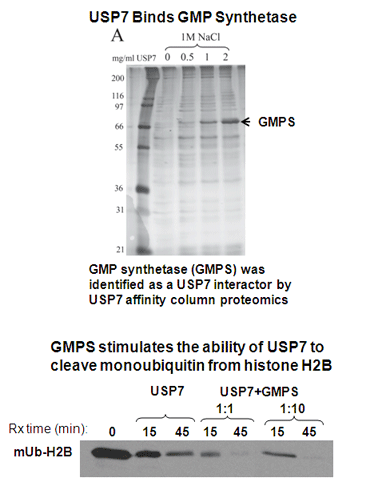
Sarkari, F., Sanchez-Alcaraz, T., Wang, S., Holowaty, M.N., Sheng, Y. and Frappier, L. 2009 EBNA1-mediated Recruitment of a Histone H2B Deubiquitylating complex to the Epstein-Barr virus Latent Origin of DNA Replication. PLoS Pathogens, 5(10):e1000624. PMID: 19834552
Sivachandran, N., Sarkari, F. and Frappier, L. 2008 Epstein-Barr Nuclear Antigen 1 Contributes to Nasopharyngeal Carcinoma through disruption of PML Nuclear Bodies. PLoS Pathogens, 4(10): e1000170. doi:10.1371/journal.ppat.1000170.
Sheng, Y., Saridakis, V., Sarkari, F., Duan, S., Wu, T., Arrowsmith, C.H. and Frappier, L. 2006 Molecular recognition of p53 and Mdm2 by USP7/HAUSP. Nature Structural and Molecular Biology. 13, 285-291
Saridakis, V., Sheng, Y., Sarkari, F., Holowaty, M.N., Shire, K., Nguyen, T., Zhang, R.G., Liao, J., Lee, W., Edwards, A.M., Arrowsmith, C.H. and Frappier, L. 2005 Structure of the p53-binding domain of HAUSP/USP7 bound to Epstein-Barr Nuclear Antigen 1: Implications for EBV-mediate immortalization. Mol. Cell 18, 25-36.
Holowaty, M.N., Sheng, Y., Nguyen, T., Arrowsmith, C. and Frappier, L. 2003. Protein interaction domains of the ubiquitin specific protease, USP7/HAUSP. J. Biol. Chem. 278, 47753-47761.
Holowaty, M.N., Zeghouf, M., Wu, H., Tellam, J., Athanasopoulos, V., Greenblatt, J. and Frappier, L. 2003. Protein profiling with Epstein-Barr nuclear antigen 1 reveals an interaction with the herpesvirus associated ubiquitin specific protease, HAUSP/USP7. J.Biol. Chem., 29987-29994.