Prion Basics - General Information about Prion Diseases
Section Navigator> General>
> Homepage
> Prion Basics
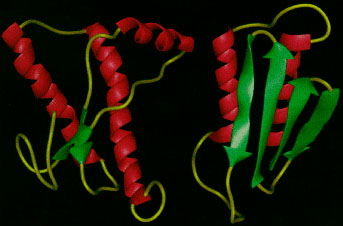
Figure 1: Modified schematic diagram of the 3-dimensional structure of normal glycoprotein PrpC (left) into its protease resistant isoform PrpSC (right) (adapted from Wikipedia (1))
Prion disease, also known as Transmissible Spongiform Encephalopathy (TSE), is a type of fatal neurodegenerative disease (2). TSE can be transmitted between humans and animals (3). Although the symptoms vary in distinct TSE diseases, they all have observable changes such as a large degree of neuronal loss, formation of spongiform, conversion of the normal glycoprotein PrpC into its protease resistant isoform known as PrpSC and accumulation of PrpSC (2). The disease can incubate in the body for many years before any signs are present. Due to inherent nature of these Prion diseases, we may witness high mortality decades from now. Prion diseases infect both animals and humans. The Prion disease for cows, deer, sheep and human are Bovine Spongiform Encephalopathy (BSE), Chronic Wasting Disease (CWD), Scrapie, and Creutzfeldt-Jakob Disease (CJD) respectively (3).
PrpC can undergo conformational changes to generate PrpSC in spontaneous or facilitated conversion via cellular factors (2). At present, the identity of the infectious material to Prion disease remains unclear (2). There are two on-going hypotheses of neurotoxicity in Prion disease. The protein-only hypothesis suggests that pathogenesis is attributed to PrpSC formation and its accumulation (3). The alternative hypothesis suggests that RNA- PrpC oligomers are the real infectious material and PrpSC does not contribute to neurotoxicity (3, 4). Unraveling the mystery behind Prion protein conversion mechanism and the cause of neurotoxicity will help in the development of better diagnostic tools and treatments to combat Prion diseases.
References:
(1) Wikimedia Inc. [Internet] Wikipedia (US): http://en.wikipedia.org/wiki/Prion_protein. 2007 Mar-[cited 2007 Mar 12] Available from: http://en.wikipedia.org/wiki/Prion_protein
(2) Supattapone S. Prion protein conversion in vitro. J. Mol. Biol. 2004; 82: 348-356
(3) Nandi PK. Prions at the crossroads: the need to identify the active TSE agent. BioEssay 2004; 26:469-473
(4) Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Depleting Neuronal Prp in Prion Infection Prevents Disease and Reverses Spongiosis. Science 2003; 302:871-874

