Issues - Ongoing Theories about Prion Diseases
Prion protein conversion
Spontaneous vs. facilitated Prion protein conversion
Individuals or animals with Prion disease has observable changes such as large degree of neural loss, formation of spongiform and conversion of the normal glycoprotein PrpC into its protease resistant isoform known as PrpSC (1). PrpC can undergo conformational changes to generate PrpSC in spontaneous or facilitated conversion via cellular factors. In vitro, purified PrpC can be converted to a PrpSC-like molecule, PrpRes, spontaneously in the presence of 50 fold molar excess of PrpRes at pH 6-8 (2). In contrast to the protease sensitive, alpha helices-dominated PrpC, PrpRes is biochemically and structurally similar to PrpSC. Both PrpRes and PrpSC are protease resistance and consist of beta sheet predominately (~43% beta, 30% alpha) (3). Due to the large amount of excess PrpRes required in spontaneous conversion, this inefficient process leads to the hypothesis that PrpRes conversion may be facilitated by cellular factors (4). It has been shown that both recombinant prp and prp brain homogenates conversion into PrpSC were facilitated in the presence of salt, chaperone proteins and sulfated glycans (5). Recent experiments with RNA extracts from mammalian brain homogenates and shsRNA (Small, highly structural, artificial RNA) have suggested that RNA is another facilitator in Prion protein conversion (2).
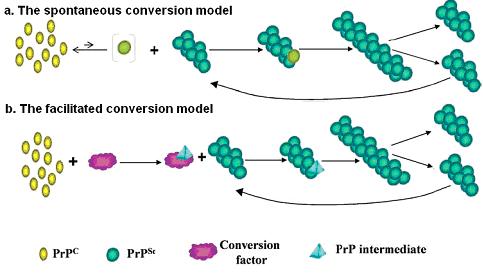
Figure 1: Different models for Prion protein conversion. (adapted from Abid K, Soto C. (3)) ( a) The spontaneous conversion model proposes that PrpC can undergo spontaneous conversion to PrpSC in the presence of PrpSC (b ) The facilitated conversion model proposes that PrpC undergoes conversion to PrpSC in the presence of facilitators and PrpSC.
References:
(1) Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Depleting Neuronal Prp in Prion Infection Prevents Disease and Reverses Spongiosis. Science 2003; 302:871-874.
(2) Deleault NR, Lucassen RW, Supattapone S. RNA molecules stimulate Prion protein conversion. Nature 2003;425:727-720.
(3) Abid K, Soto C. The intriguing Prion disorders. Cell. Mol. Life Sci 2006; 63: 2342-2351.
(4) Supattapone S. Prion protein conversion in vitro. J. Mol. Biol. 2004; 82: 348-356
(5) Vasan S, Mong PY, Grossman A. Interaction of Prion Protein with Small Highly Structured RNAs: Detection and Characterization of Prp-Oligomers. Neurochem Res 2006; 31: 629-637.

