Reviews of current RNA based technologies used in Prion Disease Research
Section Navigator> Scientific Reviews>
> Endogenous ssRNA and shsRNA
> mRNA markers in Prion Diease
> RNA aptamers and Prion Diesase
> RNA interferance and Prion Disease
Microglia activation and levels of Hsp70 mRNA: possible markers for diagnosis of Creutzfeldt-Jakob Disease
Prion diseases like Creutzfeldt-Jakob disease infect humans and can incubate in the body for many years before any signs are present (1). As a result of this incubation period, trying to diagnose it early has been of little use since symptoms occur in late stages of the disease (2). Current diagnosis involves detecting a resistant form of Prion protein (PrP-Res), where PrP-Res is resistant to proteolysis in a test-tube assay using detergents due to abnormally folded cellular Prion protein (2, 3). A weakness is that detection is only possible when there is an abundant amount of this protein, which may mean that the disease has progressed to late stages (2). With poor diagnostic tools, decades from now we could see a high mortality rate from these Prion diseases. Thus the goal of our research is to discover novel ways to diagnosis and treat Prion disease, while using RNA technology as a tool in research. This review will explore novel markers that respond to microglia activation and levels of Hsp70 mRNA in mononuclear blood cells (MBCs) in hopes that these patterns will provide better and early detection of the Prion disease Creutzfeldt-Jakob disease (CJD).
MICROGLIA + MARKERS ACTIVATED BY MICROGLIA
Research has indicated that transmissible spongiform encephalopathies agents can be carried by antigen processing myeloid cells one of which includes brain microglia (2). Thus by examining the microglia activation, which increases transcriptional response to CJD infection, we can find markers that could be used to diagnose the disease both in the early and progressive stages (4). Early detection of these markers before the accumulation of PrP-Res can lead to possible treatment which can stop the progression of CJD (2). The following results discuss the possible markers related to microglia that are detected when CJD is inoculated into brain tissue of mice. In a study by Baker et al. (5), microglia was studied due to its role in brain injury and infection. It was found that when mice were inoculated by CJD microglia react by changing their morphology, gene expression and cytokine release (5). The patterns and the appearance of the microglia from cells of mice that were infected with FU strain of CJD showed that they had distinct phenotype and activation, which could be used for diagnosis (5). To ensure the purity of microglia, RT-PCR assay was used to detect the presence of cathepsin S and the absence of GFAP and neuron specific cells (5). It was shown that CJD infected mouse brains resulted in a 4 fold increase in microglia compared to uninfected mice and infected microglia were much larger compared to normal microglia as shown using immunoflourescent microscopy (5).
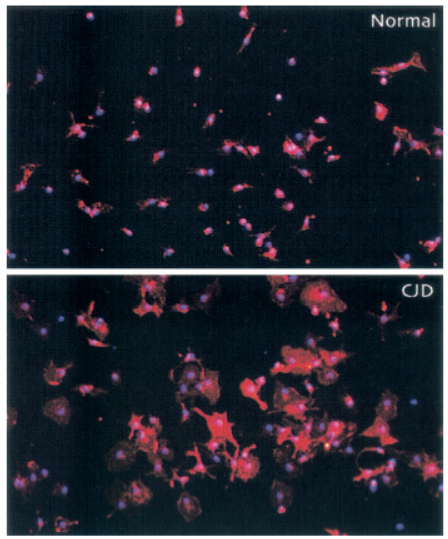
Figure 4. Modified diagram of immunoflurescence of normal and CJD-derived microglial cultures. CJD infected microglia appear to be much larger compared to microglia in normal tissue culture (Adapted from Baker CA et al. (5))
The increase in size can be explained by the activation of cathepsin S and CD68, which are two transcripts required for the function of macrophages and dendritic cells (5). It was found that cathepsin S increased 10 fold while CD68 increased 2 to 3 fold per cell in the infected microglia (5). The results from RT-PCR were measured by densitometry, and GAPDH was used as a loading control since this transcript is not affected by infection of CJD (5). RT-PCR was also used to detect cytokines and chemokines like interferon-inducible protein 10 (IP-10) and B-lymphocyte chemoattractant (BLC) because microglia secretes this in response to infectious agents (5). Tests showed an increase in mRNA levels of these in CJD-infected microglia (5). Moreover, another study using hybridization expression arrays found that there was a 5 to 20 fold increase in mRNAs of the cytokine Interleukin 1 (IL-1) (4). This experiment used cDNA expression arrays which uses small amounts of RNA extracted from microglia cells and does not require amplification, which reduces the chance of misrepresenting the levels of transcript (4). Thus, changes in size of microglia as well and the upregulation of cytokines due to the activation of infected microglia can potentially be coupled with other markers to detect CJD more accurately.
Other upregulated markers were found in the study by Lu et al. (2), which stated that microglia have a role in immune response and causes transcriptional response to CJD infection. It was also found that the transcript levels related to proteolysis, inflammatory cell recruitment and cell-cell interactions increased many folds (2). By looking at transcript levels, it was shown that there was a specific set of transcripts that were significantly elevated at early stages of CJD when there is no detectable PrP-Res protein (2). RT-PCR was used for analysis of these markers, which is an inexpensive and easy to use technique. It was found that L-selectin and serum amyloid A3 (SAA3) is expressed 20 days after inoculation (2). This was the earliest upregulation of inflammation-linked transcripts that resulted in greater than 4 fold increase compared to the normal cells (2). L-selectin and SAA3 also had a specific expression pattern that was different from other genes (2). It was shown that upregulation was higher at 20-50 days than at later stages of the disease (2). Peak levels of SAA3 occurred at 30 days, while L-selectin levels peaked at 40 days and decreased (2). Thus, the early upregulation of L-selectin and SAA3 and its specific pattern of upregulation can be utilized for early diagnosis of CJD (2).
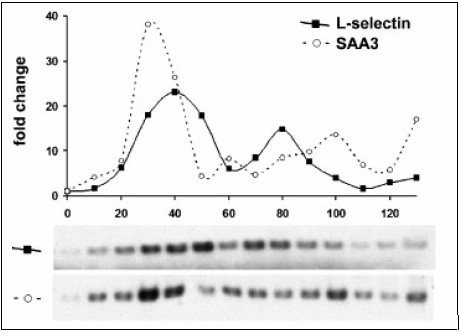
Figure 1. Modified diagram of distinct expression of L-selectin and SAA3 gene patterns during CJD progression. Graph shows SAA3 transcript levels peaking at 30 days, while L-selectin levels peak at 40 days. RT-PCR blots are used to show the fold-change versus normal brain. (Adapted from Lu ZY et al. (2))
Flow cytometry showed there was no increase in T lymphocytes positive for L-selectin and that the pattern of L-selectin was particular to infectivity of CJD (2). Moreover, on day 30, the transcripts of myeloid cell recruitment factors MIP-1a, MIP-1 Ŗ and MCP1 were induced along with IL-1 a and TNF a proinflammatory activators (2). They were upregulated 10 folds and decreased as shown on the graphs of the RT-PCR blots (2).
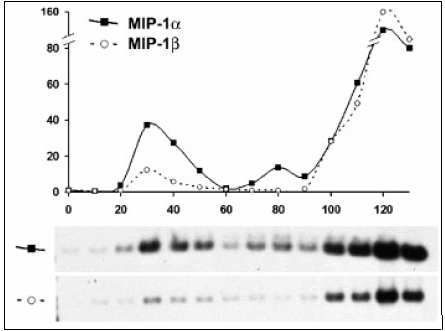
Figure 2. Modified Diagram of Distinct expression of MIP-1a and MIP-1Ŗ gene patterns during CJD progression. Graph shows an early peak at 30 days of MIP-1a and MIP-1 Ŗ indicting an increase in these transcripts. (Adapted from Lu ZY et al. (2))
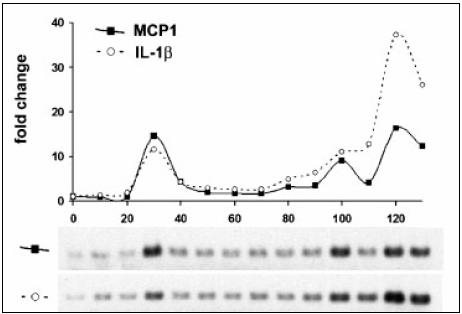
Figure 3. Modified diagram of distinct expression of MCP1 and IL-1Ŗ gene patterns during CJD progression. Graph shows an early upregulation of MCP1 transcript at 30 days. (Adapted from Lu ZY et al. (2))
A positive aspect of using RT-PCR assay is that it can detect how far infection has progressed in the brain and the results can help one distinguish between CJD and Alzheimerís since it was found that there is an abundant amount of cathepsin D mRNA in patients with Alzheimerís (2).
Using markers with unique upregulation profiles when infected with CJD can be useful for diagnosis. These markers associated with microglia activation can potentially be used for early diagnosis of the disease as well as predicating the progression of the infection by tracking their levels of upregulation. Moreover, this data shows that microglia activation in CJD involves many pathways with many associated molecules. Perhaps further research on these can potentially result in the discovery of new markers that are specific to CJD. However a general weakness of these studies is that different strains of CJD result in different pathology and microglia activation. For example in the study by Lu et al. (2), the pattern of the transcript pattern described is specific for the FU agent, while a different pattern resulted from brains infected with SY sporadic agent. Moreover, these studies were conducted on mice inoculated with CJD strains, the human is much more complex and other factors like pollution and chemical exposure from the environment may not yield the same patterns. More studies must be done to address these issues.
HSP70 mRNA levels
Another marker that can be examined for the diagnosis of CJD is heat shock 70 (HSP70). In the study by Shyu et al. (6), the researchers examined the effectiveness of measuring the expression of Hsp70 mRNA in MBCs from patients diagnosed with CJD as a means of a noninvasive way of diagnosing the Prion disease. From Northern blotting, it was found that the levels of Hsp70 mRNA relative to GAPDH mRNA were significantly higher in CJD patients compared to patients with neurodegenerative diseases that are not transmissible (6). A possible explanation of this increase in mRNA is due to the infection which causes a misfolded protein response. After stress like CJD infection, there is a rapid increase in Hsp70 transcription (7). Eventually the number of denatured polypeptides is reduced and HSP70 protein accelerates the degradation of its own mRNA (7). However, in the case of CJD we find that Hsp70 mRNA increases which suggests that the number of denatured polypeptides is not being cleared. Northern blotting is able to detect Hsp70 mRNA in MBCs, which is a noninvasive method to diagnose CJD (6).
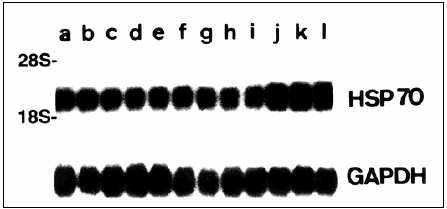
Figure 5. Modified diagram of a Northern blot of MBC from patients. Lanes j-l are from patientsí with CJD. (Adapted from Shyu WC et al. (6))
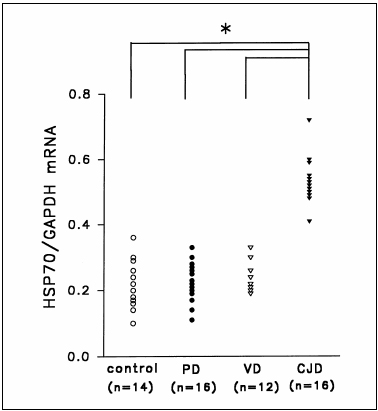
Figure 6. Modified diagram of Plot for HSP70: GAPDH mRNA level in patient with CJD and other neurodegenerative disease. Patients with CJD show an elevated level of HSP70 mRNA (Adapted from Shyu WC et al. (6))
However, further tests need to be performed to differentiate between patients with CJD and neurodegenerative diseases like Alzheimerís disease (6). Thus Hsp70 mRNA in MBCs can potentially become a useful marker for the diagnosis of CJD since detection of this infection in the blood is the most sensitive and non invasive diagnostic tool, however more tests need to be performed to determine if it is able to diagnose CJD early, as these test results are based on patients already diagnosed with CJD (2, 6).
In conclusion, markers like L-selectin, SAA3, myeloid cell recruitment factors MIP-1a, MIP-1 a and IL-1 and levels of Hsp70 mRNA in MBCs have been shown, using RT-PCR or northern blotting, to be upregulated by mice inoculated with CJD and Hsp70 mRNA in patients diagnosed with CJD. These markers appear to be better diagnostic tools compared to the conventional detection of PrP-Res as some markers are upregulated even before Prp-Res is detected (2). This early detection means early administration of treatments and lower mortality rates. However, more studies of these markers and their patterns must be performed using different virulent strains of CJD to ensure its effectiveness and relevance to the population. Detecting levels of Hsp70 mRNA in the blood of infected patients is also a reliable way to diagnose CJD in a noninvasive way, but more studies of early diagnosis needs to be done in this area. It is clear from the research that new markers are being discovered constantly, and these markers are important for developing specific upregulation patterns for CJD which can lead to early detection and better diagnostic tools to prevent the spread of this Prion disease.
References
(1) Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nature. 2006; 4: 201-211.
(2) Lu LY, Baker CA, Manuelidis L. New Molecular Markers of Early and Progressive CJD Brain Infection. Journal of Cellular Biochemistiry. 2004; 93: 644-652.
(3) Marella M, Chabry J. Neurons and astrocytes respond to Prion infection by inducing microglia recruitment. The Journal of Neuroscience. 2004; 24(3): 620-627.
(4) Baker CA, Manuelidis L. Unique inflammatory RNA profiles of microglia in Creutzfeldt-Jakob disease. PNAS. 2003; 100(2): 675-679.
(5) Baker CA, Martin D, Manuelidis L. Microglia from Creutzfeldt-Jakob disease-infected brains are infectious and show specific mRNA activation profiles. Journal of Virology. 2002; 76(21):10905-10913.
(6) Shyu W, Kao M, Chou W, Hsu Y, Soong B. Creutzfeldt-Jakob disease: heat shock protein 70 mRNA levels in mononuclear blood cells and clinical study. J Neurol. 2000; 247: 929-934.
(7) Blakrishnan K, Maio AD. Heat shock protein 70 binds its own messenger ribonucleic acid as part of a gene expression self-limiting mechanism. Cell Stress and Chaperones. 2006; 11(1): 44-50.

