Reviews of current RNA based technologies used in Prion Disease Research
Section Navigator> Scientific Reviews>
> Endogenous ssRNA and shsRNA
> mRNA markers in Prion Diease
> RNA aptamers and Prion Diesase
> RNA interferance and Prion Disease
RNA’s role in Prion protein conversion and RNA-PrpC oliogmers’ contribution to neurotoxicity
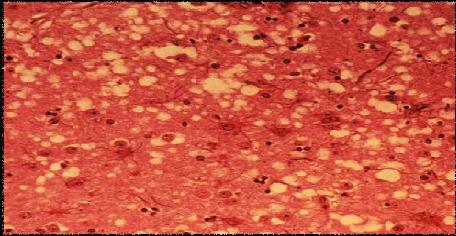
Figure1. Modified picture showing spongiform formation in brain’s grey matter as a result of PrpSC accumulation in Prion disease (adapted from U.S. Department of Agriculture website (1))
This review examines the investigative approaches used to elucidate the structure of RNA facilitator to Prion protein conversion, the practical application of endogenous RNA in the amplification of PrpSC-like compound PrpRes, the use of small, highly structured, artificial RNA (shsRNA) to study the proposed RNA induced-Prion protein conversion and possible neurotoxicity attributed to RNA-PrpC oligomers formation.
RNase A and RNase T1 inhibit PrpRes amplification
The investigation of Prion protein conversion can be done under a cell free system using protein modified misfolding cyclic amplification (mPMCA) (2). This technique does not involve sonication and thus can amplify PrpRes in its intact, undenatured form (2). Deleault et al. showed that PrpRes amplification from a mixture of normal hamster homogenate and PrpSC was inhibited by the addition of purified RNase A and RNase T1; in which both cleave single stranded RNA (3). Other nucleases such as DNase and heparinase did not show inhibition to PrpRes amplification (3). RNase VI and RNase H, specific for the double stranded RNA and DNA/RNA complex cleavage respectively, did not inhibit PrpRes amplification (3). Therefore, these collective results suggest that single stranded RNA is responsible for stimulating the conversion of PrpC to PrpRes conversion and amplification (3). This approach of using RNase and other nucleases with different specificities is a good way to narrow down structure of the facilitator to Prion protein conversion. If single stranded RNA also induces Prion protein conversion in vivo, technique using RNase A and T1 to cleave single stranded RNA may serve as a possible therapeutic tool to down regulate PrpC-PrpSC conversion.
Prion protein conversion is induced by single stranded RNA
Recent experiments with RNA extracts from mammalian brain homogenates have suggested that RNA is one of the facilitators in Prion protein conversion (3). mPMAC was adopted to study the mechanism of PrpRes conversion. The addition of RNA extract from hamster brain homogenates stimulated PrpRes amplification from mPMAC by 24 fold compared to only 6 fold of increase in the absence of RNA addition (3). This finding provides evidence that RNA might be one of the conversion factors. By increasing the rate of PrpRes amplification, addition of single strand RNA extracts in combination with PMCA method can serve as a more sensitive diagnostic test for Prion diseases (2). Although this finding cannot confirm interaction between RNA and PrpC in vivo, it provides evidence in supporting the RNA-induced Prion protein conversion hypothesis.
The use of small, highly structured, artificial RNA (shsRNA) to study Prion protein conversion
With the supporting results that endogenous single stranded RNA extract stimulates PrpRes amplification, single stranded shsRNA was artificially constructed via T7 RNA polymerase in vitro to further study the role of RNA in Prion diseases (4). Gel shift assay results show high affinity of shsRNA RQ11+12 (197 nucleotide) to protease sensitive human recombinant prp protein (PrpSen) in the presence of bovine calf serum (BCS) under physiological pH (5). The presence of PrpSen in this ribonuceloprotein was confirmed and determined by PrpSen specific- antibody (5). RQ 11+12 also formed ribonucleoprotein with PrpC in hamster, mouse and rat’s brain homogenates, indicating RQ11+12 can also bind to endogenous PrpC (5). The protease K protection assay indicated that these ribonucleoproteins in the presence of BCS are protease K resistant just like PrpRes (5). In the absence of BCS, ribonucleoprotein was prone to protease K digestion (5). This finding suggests that components within BCS are essential to for the conversion of PrpSen to its protease-resistant form (5). This is not a surprising result because prior experiments have shown that other cellular factors are also needed for Prion protein conversion (5). The approach of using shsRNA further supports the theory that single stranded RNA is one of the cellular factors facilitating the conversion of PrpSen into a protease resistance complex in vitro. Since shsRNA is a synthetic RNA, its role as a facilitator should be further analysed by examining structural similarity between shsRNA RQ 11+12 and the endogenous single stranded RNA extract that promote PrpRes amplification.
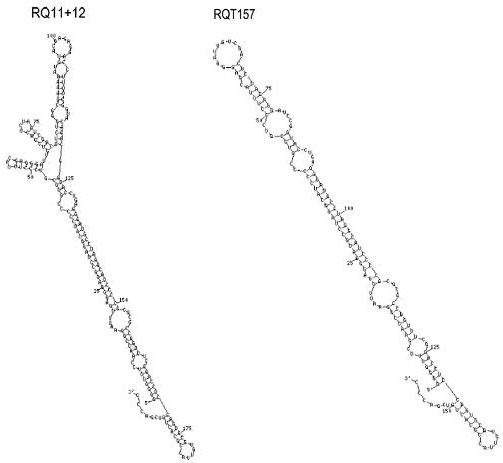
Figure 2. Modified diagram of shsRNAs’ structures: RQ11+12 and RQT-157 (adapted from Vasan S et al. (4))
The formation of toxic RNA-prp oligomers in vitro via shsRNA
The formation and aggregation of PrpSC has been the commonly accepted cause for Prion disease (6). However, Mellucci et al showed PrpSC may not be contributing to neurotoxicity as PrpC depletion can reverse early Prion disease stage despite PrpSC replication in vitro (4, 7). Another study characterized PrpC -oligomers in Scrapie-infected brain (4). Therefore, it is suggested that an intermediate form, possibly the soluble, protease sensitive PrpC-RNA oligomers, maybe the initiators to neurotoxicity (7). Ovine recombinant PrpC prp incubated in shsRNA RQ 11+12 or RQT 157 in the absence of serum factors yielded a high void volume elution (>150KD) from size chromatography compared to a low void volume elution (~40KD) in Tris-HCl buffer incubation (4). These findings indicate the formation of shsRNA-ovine prp oligomers. ShsRNA-ovine prp oligomers formation in the 150KD elution was confirmed by the modified ELISA, which detected high numbers of ovine PrpC epitopes with capture and reporter antibodies (4). The Mitoscan Electron Transfer assay showed that the shsRNA-ovine prp oliogmers caused inhibition of electron transport, a sign of toxicity (4). This approach of using shsRNA to induce the formation of PrpC-shsRNA oligomers has provided a model to test for the neurotoxicity of RNA-prp oligomers in vitro. However, the fact that shsRNA-ovine PrpC oligomers formation only occurs in the absence of serum factor raises questions as to whether in this vitro finding is a good representation of the in vivo condition.
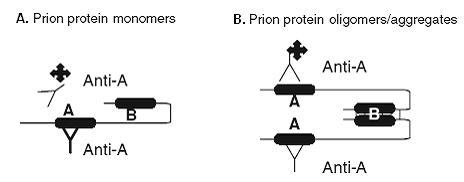
Figure 3: Modified schematic diagram modified ELISA method to verify formation of shsRNA-ovine prp oligomers. (adapted from Vasan S et al. (4)) (A) For monomer, the use Anti A as both the capture and reporter antibody to detect epitope on Prion protein monomer. The reporter has no binding and does not result in signal detection. (B) The use of Anti A as both the capture and reporter antibody to detect epitopes on Prion protein oligomer. Reporter binding with oligomer will result in signal detection.
Conclusion
The addition of RNase or endogenous RNA extract from brain homogenates in combination with modified PMCA has confirmed the facilitating role of single stranded RNA in Prion protein conversion in vitro (2,3). The practical application to this finding is the addition of single stranded RNA extract to increase PrpRes amplification, and thus, increasing the sensitivity in Prion disease detection (2). The application of shsRNA provides further evidences that RNA may be a facilitator of Prion-protein conversion (5). Furthermore, shsRNA- ovine prp oligomers formation in the absence of serum allows us to test for neutrotoxicity of this intermediate complex in the facilitated Prion protein conversion model (7). Although the ultimate cause of Prion diseases remains unclear, all these findings have provided insights in Prion protein conversion and possible causes of Prion disease pathogenesis. The next step will be to investigate whether specific single stranded RNA plays a facilitating role in Prion protein conversion or RNA-prp oligomers formation in vivo.
References:
(1) United States Department of Argriculture Animal and Plant Health Inspection Service [Internet]. BSE Photo Gallery(US)-2007 March- [cited March 12] Available from: http://www.aphis.usda.gov/newsroom/hot_issues/bse/index.shtml.
(2) Supattapone S. Prion protein conversion in vitro. J. Mol. Biol. 2004; 82: 348-356 Deleault NR, .
(3) Lucassen RW, Supattapone S. RNA molecules stimulate Prion protein conversion. Nature 2003;425:727-720.
(4) Vasan S, Mong PY, Grossman A. Interaction of Prion Protein with Small Highly Structured RNAs:.
(5) Detection and Characterization of Prp-Oligomers. Neurochem Res 2006; 31: 629-637.
(6) Akder VM, Zeiler B, Kryukov V, Kascsak R, Rubenstein R, Grossman A. Small, Highly Structured RNAs Participate in the Conversion of Human Recombinant PrpSen to PrpRes in Vitro. J. Mol. Biol. 2003; 332:47-57.
(7) Nandi PK. Prions at the crossroads: the need to identify the active TSE agent. BioEssay 2004; 26:469-473.

